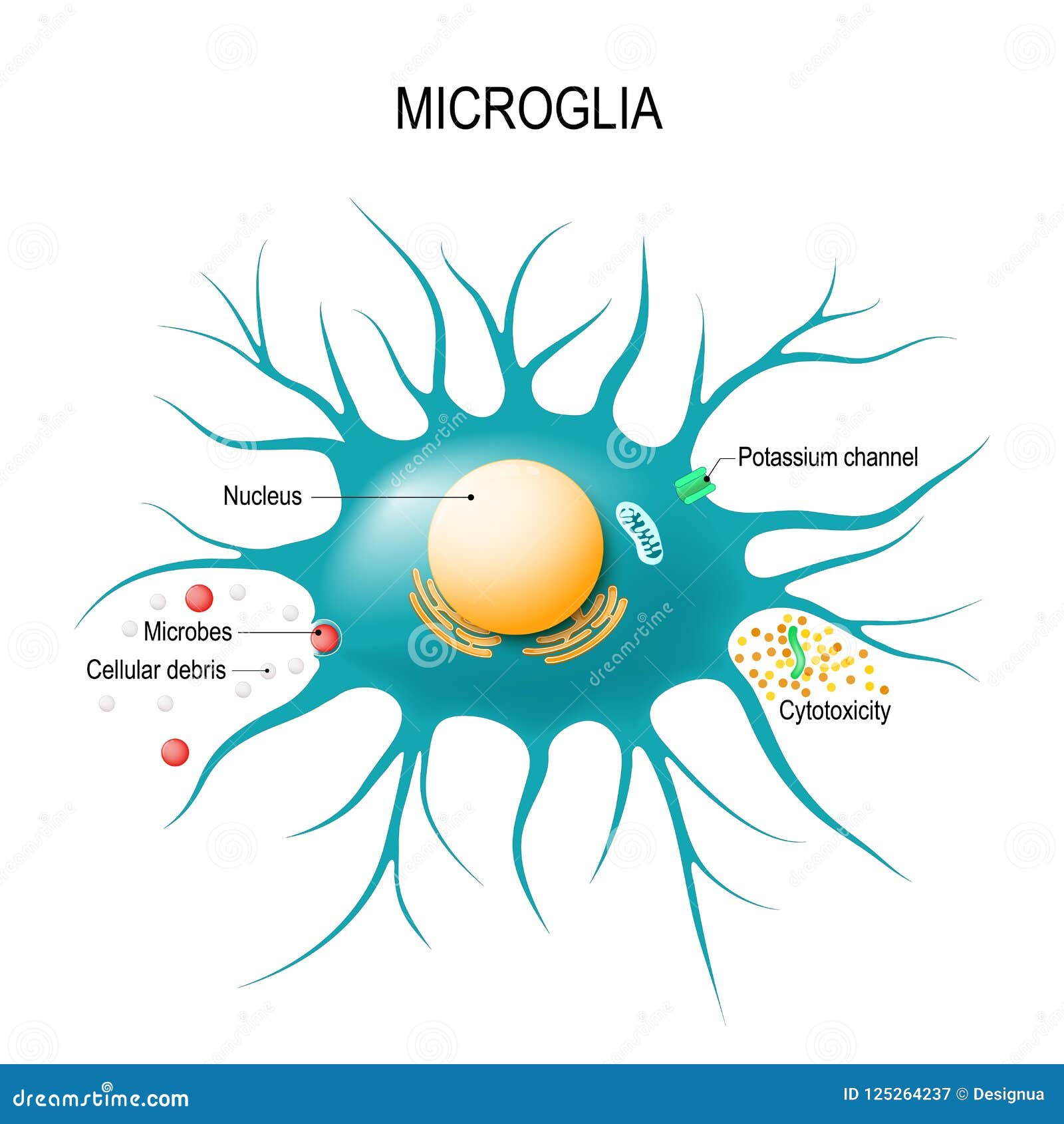
Microglial cells are the brain’s resident immune warriors, essential in maintaining neural health and function. These specialized glial cells constantly monitor the brain’s environment, responding to injury, infection, and neurodegeneration. In the context of Alzheimer’s disease, microglia play a pivotal role; they not only clear out dead cells but also engage in synaptic pruning, a process that can become detrimental when misregulated. Research led by notable neuroscientist Beth Stevens has highlighted how dysfunctional microglial activity contributes to neurodegenerative diseases, paving the way for innovative diagnostics and therapies. As the population ages, understanding the implications of microglial cells becomes increasingly critical for improving the lives of millions affected by Alzheimer’s and related disorders.
Often referred to as the brain’s immune system, microglial cells are vital for maintaining neurological health and responding to damage. These versatile cells play key roles in supporting synaptic plasticity and are involved in clearing cellular debris, which is crucial in the context of conditions like Alzheimer’s disease. The groundbreaking research by scientists like Beth Stevens emphasizes the double-edged sword nature of microglial activity; while they assist in neuroprotection, their improper functioning can lead to severe ramifications in neurodegenerative diseases. Additionally, the process of synaptic pruning, an essential developmental function, can sometimes shift towards pathological outcomes in aging individuals. Understanding these phenomena is crucial for developing therapies targeting these brain immune cells to combat disorders like Alzheimer’s and enhance therapeutic strategies.
Understanding the Role of Microglial Cells in Alzheimer’s Disease
Microglial cells are essential components of the brain’s immune system, playing a pivotal role in maintaining neural health. These specialized cells serve as guardians of the brain, constantly monitoring for any signs of injury or disease. By engaging in processes such as synaptic pruning, microglia help ensure that neural circuits function efficiently. However, a shift in their normal functioning can lead to detrimental outcomes, particularly in neurodegenerative diseases like Alzheimer’s. Aberrant synaptic pruning by microglial cells has been implicated in the progression of Alzheimer’s, where they may mistakenly eliminate essential neuronal connections, thereby exacerbating cognitive decline.
Research, such as that conducted by Beth Stevens, underscores the significance of understanding microglial functionality in the context of Alzheimer’s disease. Her studies reveal that when microglial cells malfunction, they can inadvertently contribute to the pathology associated with neurodegenerative diseases. As we delve deeper into the molecular mechanisms of microglial action, we uncover potential targets for therapy that could slow down or even halt the progress of Alzheimer’s. This insight is crucial, given that millions are affected by this condition, emphasizing the need for innovative strategies that harness the brain’s immune system to combat illness.
The Impact of Aberrant Synaptic Pruning on Neurodegenerative Diseases
Synaptic pruning is a normal developmental process where unnecessary synapses are eliminated, allowing the brain to adapt and learn more effectively. However, when this process goes awry, it can contribute to various neurodegenerative diseases, particularly Alzheimer’s. Aberrant pruning has been shown to remove synapses that are still critical for memory and cognitive function, leading to the symptoms observed in patients with Alzheimer’s. This disruption highlights the importance of precise regulatory mechanisms governing microglial activity to ensure that synaptic health is preserved throughout the lifespan.
The implications of Stevens’ research extend beyond understanding Alzheimer’s; they shed light on the underlying mechanisms of other neurodegenerative conditions like Huntington’s disease. By identifying biomarkers linked to abnormal microglial activity and synaptic pruning, researchers can potentially develop targeted therapies to reverse or mitigate the effects of these disorders. Such advancements could radically change the landscape of treatment for neurodegenerative diseases, bringing hope to millions suffering from conditions that currently have no cure.
Revolutionizing Neurological Research Through Basic Science
Beth Stevens’ research exemplifies the transformative power of basic science in unraveling the complexities of the brain. The exploration of microglial cells and their functions has opened up new avenues for understanding neurodegenerative diseases like Alzheimer’s. The progression from curiosity-driven experiments to discoveries with significant clinical implications showcases how foundational research can lead to breakthroughs in medicine. By connecting basic scientific principles with practical applications, researchers can develop innovative approaches to combat neurodegenerative diseases that devastate countless lives.
Moreover, the support from federal agencies, particularly the National Institutes of Health, plays a crucial role in fostering such transformative research. Funding enables scientists like Stevens to pursue their inquiries without immediate pressure for tangible outcomes. This investment in basic science is critical for cultivating the next generation of researchers who can advance our understanding of the brain and its maladies. As seen in Stevens’ work, the ability to explore fundamental questions can yield insights that dramatically improve the development of therapies for widespread neurological conditions.
Potential Biomarkers for Alzheimer’s Disease Revealed by Microglial Research
The identification of potential biomarkers for Alzheimer’s disease has been greatly influenced by recent findings in microglial research. As microglial cells are involved in synaptic pruning and maintaining brain homeostasis, their activity patterns can provide insight into the early stages of neurodegeneration. By analyzing the behavior and alterations in microglial cells, researchers are hoping to develop biomarkers that can indicate the onset of Alzheimer’s even before clinical symptoms manifest. This early detection could revolutionize how we approach the treatment and management of the disease.
Through the groundbreaking work of researchers like Beth Stevens, advancements in biomarker discovery can lead to more effective monitoring of Alzheimer’s progression. If we can detect the disease at its nascent stages, treatment options could be tailored more effectively, potentially improving outcomes for those at risk. The integration of microglial health into diagnostic procedures marks a significant step forward in the fight against neurodegenerative disease, proving that understanding the immune system of the brain can yield critical insights into its functional decline.
The Future of Neurodegenerative Disease Treatment: Insights from Microglial Activity
As researchers continue to explore the world of microglial cells, the future of neurodegenerative disease treatments appears promising. Understanding the mechanics of how these cells contribute to diseases like Alzheimer’s provides the foundation for developing new therapeutic interventions. Innovative approaches could involve harnessing microglial activity to restore synaptic health, rather than merely reacting to symptoms. This proactive strategy underscores the importance of viewing microglia as vital players in neurodegenerative disease pathology and potential treatment pathways.
The implications of these insights stretch beyond Alzheimer’s, suggesting a paradigm shift in how we approach all neurodegenerative disorders. By fostering a deeper exploration of microglial functionality, we can uncover processes that not only prevent disease development but also promote recovery and repair. The pursuit of therapeutics informed by microglial research may lead to groundbreaking treatments that address the root causes of neurodegeneration, offering hope to millions affected by these devastating conditions.
The Importance of Collaborations in Alzheimer’s Research
Collaborative efforts in Alzheimer’s research, such as those seen at the Stevens Lab, highlight the significance of interdisciplinary approaches in addressing complex neurodegenerative diseases. Scientists from various fields come together to share knowledge and techniques, allowing for a comprehensive understanding of brain function and pathology. Such collaborations can accelerate the discovery of effective treatments by merging expertise on microglial cell behavior, synaptic health, and the molecular mechanisms underlying Alzheimer’s disease.
With increasing availability of advanced technologies and methodologies, researchers can analyze data more efficiently, leading to a faster identification of promising therapeutic targets. Collaborative research environments also foster innovation, as different perspectives can inspire new hypotheses and experimental designs. By continuing to build on these partnerships, the scientific community stands a better chance of uncovering effective solutions to combat Alzheimer’s and other neurodegenerative diseases that plague society.
The Evolution of Understanding the Brain’s Immune System
The evolution of our understanding of the brain’s immune system, particularly with respect to microglial cells, represents a major shift in neuroscience. Historically, microglia were thought to play a minimal role in brain function, but emerging research reveals their fundamental importance in both brain maintenance and the response to neurodegeneration. This reevaluation is crucial as it highlights these cells not merely as defenders against disease but as active participants deeply involved in brain architectural changes, which can lead to new therapeutic avenues.
Studies spearheaded by researchers like Beth Stevens emphasize that understanding the role of microglial cells in synaptic pruning and response to neurodegeneration paints a clearer picture of how the immune system interacts with neural networks. As we refine our models of brain immunity, we can better understand the etiology of diseases like Alzheimer’s, ultimately leading to more targeted and effective treatments. As knowledge progresses, so too does the potential for advancements in neurological health and well-being across populations.
Exploring Synaptic Pruning as a Gateway to New Treatments
Exploring the mechanisms of synaptic pruning offers a potential gateway to innovative treatments for neurodegenerative diseases. The process itself is critical for normal brain development and function, but when dysregulated, it can contribute to the pathology of Alzheimer’s disease. By utilizing insights from studies on microglial cells, researchers can develop strategies aimed at controlling and regulating synaptic pruning to prevent destructive outcomes. Investigating these pathways provides a new perspective on how we can maintain cognitive health in aging populations.
Furthermore, understanding the nuances of synaptic pruning allows us to identify points of intervention that could mitigate diseases like Alzheimer’s. For instance, therapies aimed at regulating microglial activity during the pruning process could potentially safeguard viable synapses, supporting cognitive functions even in the face of neurodegeneration. As research progresses, the focus on synaptic health through the lens of microglial function may lead to pioneering therapies that not only address symptoms but also enhance brain resilience against age-related decline.
The Long-term Vision for Alzheimer’s Disease Research
The long-term vision for Alzheimer’s disease research is one that emphasizes prevention over cure, leveraging the insights gained from microglial and synaptic pruning studies. With a clearer understanding of how the brain’s immune system operates and contributes to neurodegeneration, future research can focus on early interventions that forestall the onset of Alzheimer’s. This shift in focus is essential for improving the quality of life for millions of individuals and their families who face the challenges posed by this disease.
By investing in longitudinal studies that track the health of microglial cells and their impact on cognition, researchers can create a holistic understanding of Alzheimer’s progression. This perspective allows for the development of preventative therapies that could be administered even before the onset of the disease. As researchers like Beth Stevens continue to lead the charge in uncovering the relationship between the brain’s immune responses and cognitive function, the horizon for Alzheimer’s research expands, promising a future with more effective strategies for combating this pressing public health issue.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease?
Microglial cells are vital components of the brain’s immune system, playing a crucial role in Alzheimer’s disease by monitoring brain health, clearing out dead or damaged cells, and participating in synaptic pruning. Aberrant pruning by microglia has been linked to the progression of Alzheimer’s, leading to synaptic loss and neurodegeneration.
How are microglial cells related to neurodegenerative diseases?
Microglial cells are implicated in various neurodegenerative diseases, including Alzheimer’s and Huntington’s disease. They help maintain brain homeostasis but can contribute to disease when their activity becomes dysregulated, particularly through excessive synaptic pruning, which can lead to neuronal damage and cognitive decline.
What is synaptic pruning and its connection to microglial cells?
Synaptic pruning is a process where microglial cells remove excess or weak synapses during neural development and remodeling. While this process is essential for healthy brain function, dysfunctional microglial activity can result in excessive pruning, contributing to neurodegenerative diseases such as Alzheimer’s.
How has Beth Stevens’ research advanced our understanding of microglial cells?
Beth Stevens’ research has advanced our understanding of microglial cells by highlighting their functions in synaptic pruning and their role in neurodegenerative diseases like Alzheimer’s. Her findings suggest that disturbances in microglial activity could lead to synaptic loss, offering new avenues for potential biomarkers and treatments.
What impact do microglial cells have on brain health?
Microglial cells are essential for brain health as they act as the immune defenders, clearing debris and supporting neuronal health. Their ability to prune synapses optimally is critical for normal brain function. However, dysregulation of these processes can lead to conditions like Alzheimer’s disease, indicating that microglia are at the intersection of health and disease in the brain.
Can microglial cells be targeted for Alzheimer’s treatment?
Yes, researchers are exploring how to target microglial cells for Alzheimer’s treatment by developing therapies that regulate their activity to prevent the improper synaptic pruning that can lead to neurodegeneration. This research aims to manipulate microglial function to protect neurons and improve outcomes for individuals with Alzheimer’s and other neurodegenerative diseases.
What discoveries have been made about microglial functions in the brain’s immune system?
Recent discoveries about microglial functions have revealed that these cells are not only involved in immune defense but also play a vital role in brain development and function. They are key players in synaptic pruning, which helps shape neural circuits. Dysfunctions in these processes have significant implications for neurodegenerative diseases like Alzheimer’s.
| Key Points | Details |
|---|---|
| Role of Microglial Cells | Microglial cells act as the brain’s immune system, patrolling for illness and injury. |
| Impact on Neurodegenerative Diseases | Aberrant pruning by microglia can lead to Alzheimer’s and Huntington’s diseases. |
| Research Foundation | Research by Beth Stevens is supported by NIH funding and focuses on understanding microglial behavior. |
| Importance of Basic Science | Basic science is crucial for discovering new treatments and understanding brain diseases. |
| Future Implications | Findings could lead to new biomarkers and therapies for the 7 million Americans with Alzheimer’s. |
Summary
Microglial cells play a vital role in brain health and disease prevention. As the brain’s immune system, they are crucial for clearing damaged cells and helping to prune synapses. However, improper functioning of these cells can contribute to devastating neurodegenerative diseases like Alzheimer’s. Through the innovative research of scientists like Beth Stevens, we are beginning to uncover the mechanisms behind microglial activity and their implications for disorders affecting millions. This foundational work not only enhances our understanding of brain biology but also paves the way for developing effective treatments that could improve the lives of those suffering from neurodegenerative diseases.